One: The basic properties of cermet cemented carbide
Cermet cemented carbides produced by the Powder metallurgy process and are known for their high hardness and wear resistance.
It is characterized by a very high red hardness, that is, at high temperature (900-1100 °c) to maintain the hardness of the ability, and steel at 600 °C will lose its inherent hardness.
The presence of refractory metal carbides determines these valuable properties of cemented carbides. Moreover, the chemical composition, content, and grain size of these carbides have a significant influence on the physical-mechanical properties of cemented carbides.
Only refractory metal carbides cannot be made into hard alloys. Although they are hard, they are brittle. It’s not a tool material.
To obtain sufficient strength, bonding metals must add, and the existence of hard (refractory metal carbides) and soft (cobalt) materials in cemented carbides constitutes the contradiction of this kind of thing. This kind of opposition inside the idea is the primary reason for the thing development.
Two: Refractory Metal Carbide
There is a lot of periodic tables out there with a lot of useful features:
High melting point, high strength, can with carbon, nitrogen, Boron to produce tough compounds. Tungsten, Molybdenum, titanium, vanadium, and niobium are the most widely used cemented carbides.
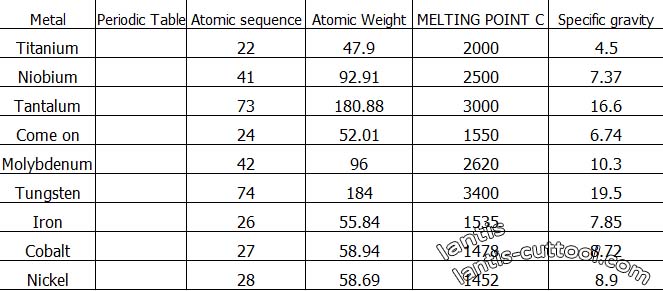
These metals and carbon compounds are an essential part of the composition of cemented carbides. These carbides are structurally called interstitial phases.
That is non-metallic atoms drilled into the lattice of metallic particles, which prevents the metal atoms from sliding, so it has a high hardness. The gap phase formed when the ratio of the atomic radius of the nonmetal atom to a metal atom is less than or equal to 0.59.
Rx / RM ≤0.59.
Interstitial phase-metal-carbon (nitrogen) compounds with similar metallic properties: metallic Luster, electrical conductivity, thermal conductivity. In most cases, it has high hardness and high melting point.
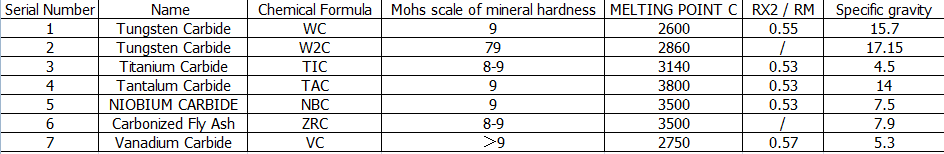
Some hard alloys made of two or more carbides. Therefore, it is of considerable significance to study the interaction between carbides and carbides.
The characteristics of the interaction between carbides mainly determined by the lattice type of the primary carbides, the temperature conditions of the communication, and the chemical composition of the materials.
The metal atoms are cubic crystal system, and the HOMOMORPHIC, refractory carbide binary alloys all form a continuous robust solution
The Tic-WC system is the most practical one. The reliable solution in WC keeps the high hardness and wear resistance of WC, and the solubility of WC in WC is very high.
The Tungsten Carbide dissolved at the same time as the titanium carbide is saturated so that the reliable solution of WC melting in TIC almost becomes an ideal TIC lattice.
In the case where Tic is low in carbon, the WC dissolved in Tic, and the WC is converted to W2C and acts as a carbon saturated Tic. This alloy of two or more carbides is called complex carbides, which are the basis of cemented carbides.
Cemented Carbide
Refractory Metal carbides have high Brittleness in addition to high hardness. Therefore, a single brittle material used to press, sintering into cemented carbide, is not feasible.
Eliminate Brittleness and maintain hardness, it is necessary to add bonding metal, the role of bonding metal is to reduce hardness and increase strength, and carbide is to increase hardness and lower resistance.
It proved from the composition of cemented carbide that the law of the unity of opposites is universal in the universe.
The development and transformation of this contradiction in cemented carbide lead to the formation of alloys of different grades, which serve industrial production.
What are the conditions for bonding metal?
- The melting point after sintering must be higher than the highest temperature when working hard alloy blade, but not too high. Otherwise, the alloy sintering is not easy to control.
- It is easy to flow after melting and has good wettability for carbide particles. Instead of a ball of mercury on the glass. Reduces the strength of the alloy.
- it shall not form brittle compounds with carbides and gaseous media during sintering.
- it shall be of reasonably high strength after sintering.
Iron Oxide: They have excellent physical and mechanical properties and can wet tungsten carbide thoroughly, but the carbide itself is Brittle, so it can not guarantee the required strength of cemented carbide.
Cobalt and nickel: All can make very good wetting tungsten carbide and has sufficient mechanical properties. Cobalt and nickel also produce unstable carbides.
Cobalt Carbide exists at 800 °C and decomposes above.
Nickel Carbide decomposes above 580 °C.
Their melting points: Cobalt 1478 °C, Nickel 1452 °C.
After sintering, Tungsten Carbide dissolved in cobalt and nickel so that the melting point will decrease. Probably around 1200 °C, but enough for a blade.
The physical properties of cobalt and nickel are excellent, especially in toughness can be fully used in cemented carbide. Thermal conductivity, electrical conductivity, is also an essential factor in cutting tools.
The higher the cobalt content, the better the impact resistance of the alloy.
Cobalt is more widely used than nickel because it is more readily available than a coin. It also proved that cobalt has better properties than metal in the actual production.
Here’s why:
1. Although nickel itself produces unstable carbides, it can interact with other carbides to form a soft, brittle material
2. Nickel powders are difficult to manufacture and are not readily available in powder as dispersed as cobalt.

Leave A Comment